how to draw molecular orbital diagram of co
Can be accommodated in the metal d orbitals. See Resources for a diagram showing the filling order.
What Is The Molecular Orbital Energy Diagram Of Co Quora
Oct 26 2016 3 min read.
. The lewis structure shows that the beryllium in beh 2 makes 2. Thanking for reading this post If you find. You can see that CO is not as it has zero unpaired electrons but NO is it has one unpaired electron.
Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na. O_2 is well-known to be paramagnetic and it is one of the successes of molecular orbital theory. The course introduces the three key spectroscopic methods used by chemists and biochemists to analyse the molecular and electronic structure of atoms and molecules.
So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. Relationship between electronic configuration and Molecular behaviour. The Aufbau principle tells you that the lowest-energy orbitals fill first but the specific order isnt sequential in a way thats easy to memorize.
Features of molecular orbital theory for metal complexes are as follows. The angular overlap diagrams for the molecular orbitals with high d orbital. Draw the orbital diagram for ion Co 2.
Sp Hybrid Orbitals in BeH2 1. We showed the following examples. Is there SP mixing in CO.
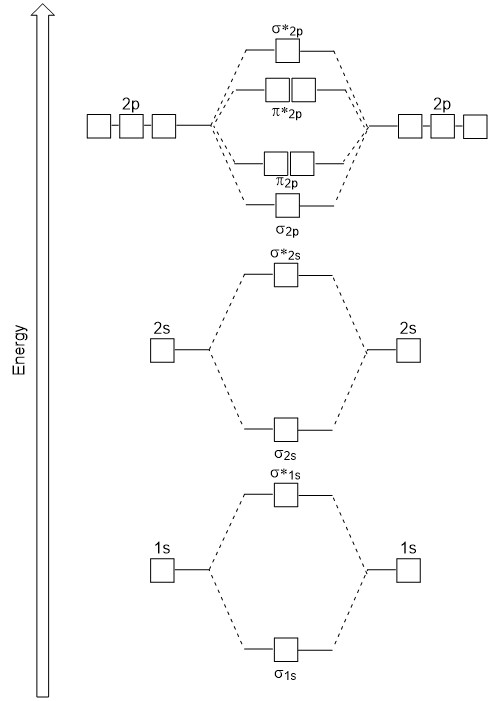
Homonuclear diatomics HF CO H3 FHF- CO 2 H2O BF3 and. Molecular Orbital Diagram of CO. The bond order is already.
In contrast to VSEPR and valence bond theory which describe bonding in terms of atomic orbitals molecular orbital theory visualizes bonding in relation to molecular orbitals which are orbitals that surround the entire molecule. The wavefunctions of properly-formed group orbitals can be deduced using the projection operator method. The valence molecular orbitals in both atoms are the 2s and 2p orbitals.
Use the buttons at the top of the tool to add orbitals in order of increasing energy starting at the bottom with the. Home Structure and Bonding Atomic Orbitals Molecular orbitals in Carbon Monoxide. Molecular Orbital Diagram of CO.
These are UVVisible Infra-red IR and Nuclear Magnetic Resonance NMR spectroscopies. Previous article Wohl-Ziegler Bromination. Thus we take 10 atomic orbitals and generate 10 molecular orbitals in accordance with the conservation of orbitals.
You have now 2 electrons left. Now mo diagrams are only simple for elements of the second row of the periodic table celi through cene. Next article Molecular Orbital Diagram of NO.
Introduction to Molecular Spectroscopy. MAKE SURE TO SUBSCRIBEThis video puts emphasis on molecular orbital diagrams a fundamental way of understanding why Diels-Alder chemistry works. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs.
NO NO NO- Is CO a Lewis acid. This approach is used only when the group orbitals are not obvious by inspection. 1The atomic orbital of the metal center and of surrounding ligands combine to form new orbitals known as molecular orbitals.
Also the molecular orbital diagram of carbon monoxide reveals that s-p mixing must be occurring since the 3σ orbital is higher in energy than the 1π orbital. Based on the amount of orbital overlap the relative changes in energy differ going from the atomic orbital to the molecular. How to draw molecular orbital diagram for heteronuclear molecules.
2The number of molecular orbitals formed is the same as that of the number of atomic orbitals combined. The applications of the MO theory extend beyond the limitations of the Valence Shell Electron Pair Repulsion VSEPR model and the Valence Bond theory. It is a linear molecule.
Bond order for NO Order by bond length. How to draw molecular orbital diagram for CO2 By signing up youll get thousands of step-by-step solutions to your homework questions. 1 If N b Nathe molecule is stable because greater number of.
Use the buttons at the top of the tool to add orbitals in order of increasing energy starting at the bottom with theOct 05 Also Cobalt orbital diagram example problem. Molecular orbitals in Carbon Monoxide. Draw the orbital diagram for the ion Co2.
There are a total of 6 electrons to add to the molecular orbital diagram 3 from boron and 1 from each hydrogen atom. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each.
Molecular Orbital Diagrams simplified. 12-12 This video describes the molecular orbital theory diagram of CO placing emphasis on how MO theory differs for homo and heteronuclear diatomics. It is a linear molecule.
Molecular Orbital MO Theory is the final theory pertaining to the bonding between molecules. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same. A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular.
The molecular orbital MO theory is a powerful and extensive approach which describes electrons as delocalized moieties over adjacent atoms. Draw the orbital diagram for ion Co 2. The molecular orbital diagram for carbon monoxide Figure 53.
A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO molecular orbital method in. Reducible representation of the orbitals in question. The first major step is understanding.
Also see here. Next well see that symmetry will help us treat larger. The purpose of MO theory is to fill in the gap for some.
Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. How to Build Molecular Orbitals. Well the MO diagram for O_2 is.
High-spin octahedral d7 has LFSE o. CONTROLS Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Watch the video solution for the question.
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. Draw the orbital diagram for the ion Co2. 1 Stability of molecules in terms of bonding and antibonding electrons.
Consider the h 2 molecule for example. Watch the video solution for the question. Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals.
The content is presented using short focussed and. All About Chemistry - July 2 2020. Note that the n 1 level only has s orbitals the n 2 level only has s and p orbitals and the n 3 level only has s p and d orbitals.
Molecular orbital diagram as a non-bonding molecular orbital. D0 ions d7 ions Fe1 Ru1 Co2.

Molecular Orbitals For Carbon Monoxide

Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube

Draw The Molecular Orbitals For Co In Order Of Energy And Fill Them With The Appropriate Number Of Electrons Label The Orbitals The Best You Can As Sigma Or Pi And As
What Is The Molecular Orbital Energy Diagram Of Co Quora

Assuming The Mo Diagram Above Would Apply To Both Species Predict How The Bond Order Will Change Upon Adding An Electron To Cn To Form Cn The Bond Order Will Increase Decrease
What Is The Bond Order Of Co Quora
8 Drawing Molecular Orbital Diagrams Flux Science
Molecular Orbital Diagram Of The Co Molecule Excluding 1s Atomic Download Scientific Diagram

Tom Teoria Do Orbital Molecular Electron Configuration Chemistry Molecule Diagram

Molecular Orbital Energy Level Diagram Of Nitrogen Oxygen Youtube
What Is The Molecular Orbital Energy Diagram Of Co Quora

Co Mo Diagram Chemistry Lessons Chemistry Classroom Teaching Chemistry
Molecular Orbitals Introductory Chemistry
Answer In Inorganic Chemistry For Lontum Rodrique 112252

Introduction To Molecular Orbital Theory Physical Chemistry Chemistry Chemistry Education

Draw The Molecular Orbital Diagram For Co Based On Your Diagram Why Does Co Always Bond Through The Carbon And Not The Oxygen Atom Study Com

Main Difference Hybrid Orbitals Vs Molecular Orbitals Chemistry Basics Chemistry Teaching Chemistry